Research
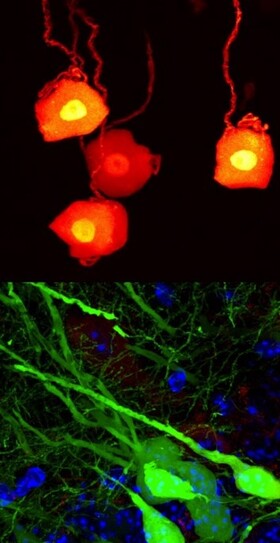
Keeping axons alive through bioenergetics and glia
Axons are the longest cellular projections of neurons relaying electrical and biochemical signals in nerves and white-matter tracts of the nervous system. As such, they are critical for neuronal wiring and transport of neuronal maintenance signals. Axons do not exist in isolation, but are inextricably and intimately associated with their enwrapping glia (Schwann cells and oligodendrocytes) to form an unique axon-glia unit.
Because of their incredible length and energetic demand (human motor neurons can be one meter long), axons are very vulnerable and at continuous risk of damage. Many debilitating neurodegenerative disorders share the common feature of early damage and demise of axons. The most relevant neurological symptoms in a number of these conditions are due to compromised axon integrity. Thus, neuroprotective therapies promoting axon stability have great potential for more efficient treatment.
The identification of such therapies requires a better understanding of the mechanisms underlying axon degeneration. Our laboratory investigates the cell-autonomous and non-cell-autonomous mechanisms of the degeneration of axons. In other words, we are attempting to elucidate what causes axon breakdown from within neurons, and which external (glial) events trigger axon loss.
Recent studies indicate that axonal degeneration is an active and highly regulated process akin to programmed cell death and occurs secondary to bioenergetic failure. Moreover, it is increasingly realized that axonal maintenance relies not only on neuron-derived provisions but also on trophic support from their enwrapping glia. The mechanism for this non-cell-autonomous support function remain unknown, but emerging evidence indicates that it is distinct form the conventional glial role to insulate axons with myelin. We are pursuing the intriguing question whether abolished support by aberrant delivery of metabolites and other trophic factors from glia into axons is mechanistically linked to the induction of axonal auto-destruction. This concept is supported by our recent findings indicating that Schwann cells shuttle glycolytic energy substrates into distressed axons, and disruption of this metabolic support function in glia promotes axon demise.
Axons are the longest cellular projections of neurons relaying electrical and biochemical signals in nerves and white-matter tracts of the nervous system. As such, they are critical for neuronal wiring and transport of neuronal maintenance signals. Axons do not exist in isolation, but are inextricably and intimately associated with their enwrapping glia (Schwann cells and oligodendrocytes) to form an unique axon-glia unit.
Because of their incredible length and energetic demand (human motor neurons can be one meter long), axons are very vulnerable and at continuous risk of damage. Many debilitating neurodegenerative disorders share the common feature of early damage and demise of axons. The most relevant neurological symptoms in a number of these conditions are due to compromised axon integrity. Thus, neuroprotective therapies promoting axon stability have great potential for more efficient treatment.
The identification of such therapies requires a better understanding of the mechanisms underlying axon degeneration. Our laboratory investigates the cell-autonomous and non-cell-autonomous mechanisms of the degeneration of axons. In other words, we are attempting to elucidate what causes axon breakdown from within neurons, and which external (glial) events trigger axon loss.
Recent studies indicate that axonal degeneration is an active and highly regulated process akin to programmed cell death and occurs secondary to bioenergetic failure. Moreover, it is increasingly realized that axonal maintenance relies not only on neuron-derived provisions but also on trophic support from their enwrapping glia. The mechanism for this non-cell-autonomous support function remain unknown, but emerging evidence indicates that it is distinct form the conventional glial role to insulate axons with myelin. We are pursuing the intriguing question whether abolished support by aberrant delivery of metabolites and other trophic factors from glia into axons is mechanistically linked to the induction of axonal auto-destruction. This concept is supported by our recent findings indicating that Schwann cells shuttle glycolytic energy substrates into distressed axons, and disruption of this metabolic support function in glia promotes axon demise.




